OPIOID USE IN CHRONIC PAINFrom The Oxford Pain Internet Site
H J McQuay DM, Clinical Reader in Pain Relief
Pain Research & Nuffield Department of Anaesthetics
University of Oxford, Oxford UK
Abstract
Many of the important questions for those who prescribe opioids in chronic
pain, and important for those who take the drugs, still have to be answered
with inadequate evidence, because we lack randomised trials on these topics.
Two of the principal reasons for 'failure' of opioids to relieve pain, incident
pain and neuropathic pain, are discussed. Some specific adverse effect problems
are addressed, and the paper concludes with a section on the vexed issue of
opioid prescription in non-cancer pain.
If we take in our hands any volume ... let us ask, "Does it contain any
abstract reasoning concerning quantity or number?" No. "Does it contain any
experimental reasoning concerning matter of fact and existence?" No. Commit it
then to the flames, for it can contain nothing but sophistry and illusion."
David Hume (1711-76)
Much of our prescribing of opioids is based on opinion rather than on evidence...
In part this is because opioid use in chronic non-cancer pain is an orphan
area, in part because trial design and conduct is not easy in this patient
group, and in part it is historic - many opioids are old drugs, and the
registration trials required for new drugs have therefore not been done. It is
remarkable how little new evidence on oral and intramuscular opioid use has
emerged since earlier reviews [1, 2, 3]. An added complication is that there is
a plethora of routes by which these drugs can be given. The fact "that it can
be done" very often pre-empts the more important question "should it be done?".
Enthusiasts for the new route carry it into practice without adequate
comparison of risk and benefit with 'established' routes. Again these arguments
have been well rehearsed [4]. Much of the 'new' evidence for spinal or
transdermal opioids does not answer the real clinical questions.
CLINICAL ASPECTS
EFFECTIVENESS
SUCCESS
Just how effective are opioids in managing chronic pain, cancer or non-cancer?
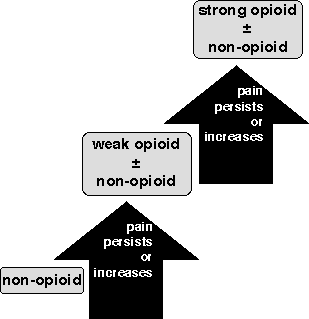
The assumption from audits of applying WHO guidelines (Figure 1) in cancer pain
is that two-thirds of patients achieve good or moderate pain relief.
The
adequacy of these audits has been challenged on process grounds [5]. In
practical terms we have refrained from detailed questioning of the adequacy of
our methods because of a wider public health political goal, which is to
achieve better coverage of the world population with implemented WHO guidelines
(and sufficient drug supplies).
In attempting to update the WHO guidelines questions emerge such as whether a
second stage is necessary and whether a fourth stage should be added [6], and
yet again there is the problem that little research evidence answers these
clinically relevant questions. Both existing guidelines and the updated
versions are based necessarily on non-optimal levels of evidence. Only 2% of
the randomised controlled trials in pain relief study cancer pain directly
[7].
Beyond trying to determine just how good the good and moderate relief is for
the two-thirds of patients, there are two obvious problem areas, neuropathic
pain and incident pain.
Failure
NEUROPATHIC PAIN
There are 2 extreme positions on opioid sensitivity. One suggests that opioid
sensitivity is a relative phenomenon and therefore that any pain can be
controlled by opioids provided that there is an adequate dose escalation and
control of adverse effects [8]. The other extreme insists that some pains are
intrinsically insensitive to opioids and that this insensitivity can be
predicted from the clinical characteristics of the pain [9]. Nociceptive pain
is thought to be sensitive to opioids while neuropathic pain is regarded as
insensitive. If neuropathic pain shows an analgesic response with opioid then
this has been attributed to mood improvement rather than to a direct effect on
pain pathways [10].
Both extremes of this controversy are supported by a very small number of
controlled trials each of which has methodological limitations. These studies
have used either single doses [10, 11], infusions of different opioids [8, 9]
or have measured pain without simultaneous assessment of adverse effects [9,
10, 11, 12]. The flaw with studies which use a single (fixed) dose or infusion
rate is that they may underestimate responses in patients with previous opioid
exposure. These patients may need more opioid to achieve analgesic effect than
the opioid naive.
Using patient-controlled analgesia (PCA) with simultaneous nurse observer
measurement of analgesia and adverse effects we gave two concentrations of
morphine in a double-blind randomised cross-over fashion and compared the
clinical responses produced by both concentrations of morphine [13].
The results did not support the assumption that neuropathic pains are always
opioid insensitive. Half of the pains judged as neuropathic achieved a good
response. Nociceptive pains, however, collectively showed a better analgesic
response, because all of them achieved a good response in at least one of the
sessions. No nociceptive pain had a poor response in this study.
Secondly, it had been suggested that the analgesic response of neuropathic
pains to opioids can be explained by the changes in mood induced by the opioids
[10]. When the results of patients with consistent responses were compared,
changes in mood reflected changes in pain intensity and relief regardless of
the clinical character of the pain, nociceptive or neuropathic. Mood improved
when pain intensity decreased or pain relief increased. No patient had a change
in mood in the absence of a change in pain intensity or pain relief, and
patients with nociceptive pains in fact showed a greater change in mood than
those with neuropathic pain. Therefore, the theory that relief of neuropathic
pains by opioids is due to changes in mood was not supported by our findings.
INCIDENT PAIN
The problem with incident pain is that the analgesia necessary to control
episodic incident pain is usually far more than is required when it is absent.
The patient between bouts of incident pain may then suffer all the adverse
effects of an 'overdose'. Optimal analgesic regimes should restore normal
function. The reality, however, is that with sub-optimal regimes, or when the
incident pain cannot be controlled satisfactorily despite an optimal regime,
function and quality of life may have to be reduced to cope with the pain. In
this delicate balance between adequate control of the incident pain and adverse
effects between times the patient often opts to 'accept' the incident pain
rather than to endure the adverse effects.
Classifying incident pain
The overlap in conventional use between the terms incident pain and
breakthrough pain is unfortunate. Breakthrough pain describes an inadequate
analgesic regime [14], and is therefore an operational definition of pain which
is not controlled, but is 'breaking through' the analgesic regime. The regime
may be adequate to control background pain but inadequate to control incident
pain.
Incident pain is used commonly to describe pain on movement. Conventionally the
widest definition is pain which comes on when the patient is not resting. This
may be pain caused by deep breathing or coughing, or pain caused by walking,
turning or lifting. These pains on activity or movement may, to an extent, be
controlled by the patient, by not moving. Incident pain thus describes some
characteristics of the pain, such as its relationship to coughing or movement,
and describes those characteristics independent of the presence or absence of
an analgesic regime. Breakthrough pain is one type of incident pain.
Complicating matters further there is a third kind of incident pain, which is
an intermittent pain which can occur even when the patient is resting, and can
occur with no known triggering process. The patient has no control over these
pains. Complete description of the pain would also include whether or not the
patient has background pain which is present at rest. The problem with
restricting the definition of incident pain to pain related to movement or to
activity is that excluding the intermittent, unpredictable pain excludes a
considerable proportion of these difficult pains (see under prevalence below).
Figure 2 summarises the definition problems.
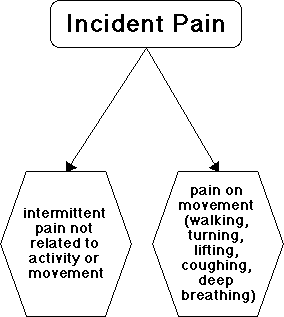
The simplest definition is that
incident pain is characterised by episodic increases in pain intensity.
Another characteristic is the patient's ability to suppress or avoid the
incident pain; the pain may be defined as "volitional" or "non-volitional"
[14]. Volitional pain should be predictable.
Characteristics of incident pain
Pain on movement may be predictable, occurring each time the patient moves in a
particular way. It may be brief or it may be prolonged. It may be acute, as a
result of a recent change in disease or due to treatment. It may be chronic.
These three axes, predictability, time course of the episodes and the
chronicity of the problem, are shown in Figure 3.
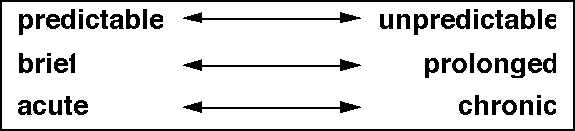
How big a problem is incident pain?
Incident pain is a big problem both because it is common and because of the
distress it causes the individual. The incidence and prevalence can only be
estimated from small surveys. Portenoy and Hagen [14] reported on 90 patients
at the Memorial Sloan-Kettering Cancer Center, and their findings are
summarised in Figure 4.
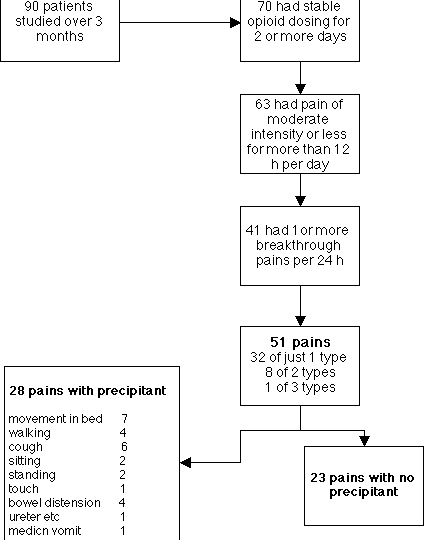
Banning et al studied 200 consecutive referrals to the
pain clinic at the Finsen Institute in Copenhagen [15]. Of the 184 patients
whose pain they evaluated 172 had pain on movement, 144 had pain at rest and
124 had their sleep interrupted by pain.
From the Danish data most patients with cancer pain will have incident pain
related to movement. Of the 131 patients with pain on movement who they
re-evaluated 1 to 2 weeks after treatment 83 still had pain on movement, 20
with moderate intensity and 36 with considerable intensity. From the American
survey (Figure 4) roughly half the patients had breakthrough pain despite an
established analgesic regime. Both these reports are from tertiary care
institutions, so that generalising to obtain incidence and prevalence data for
the general population is invalid, because patients will be referred precisely
because they do have pain. Within this selected population, however, it is a
reasonable inference that incident pain is a major problem for most patients
who have not started an 'adequate' analgesic regime, and that it remains a
major problem for half of those who are established on analgesics.
How should incident pain be managed?
Restating the problem, it is that the doses of analgesics required to control
the incident pain may be so high that toxicity results when the incident pain
is absent. Pain relief in this situation is usually relief of symptoms rather
than remedying the cause of the pain. Analgesics do not stretch to accommodate
increased severity; increased doses are required, with concomitant risk of
increased adverse effects. The questions then arise as to what changes (if any)
should be made in our 'conventional' management strategies to cope better with
incident pain, and what additional remedies should be considered.
The traditional way to manage cancer pain is illustrated by the WHO ladder
(Figure 1). Two questions need to be addressed. The first is whether or not
each of the three rungs of the ladder is able to control incident pain. The
answer required for each rung is whether or not control is feasible, given
adequate management of adverse effects. The incidence data suggests that, for a
significant proportion of patients, it is not possible. It is also possible
that better management of opioid adverse effects would allow higher opioid
doses to be used with fewer adverse effects. An open study of methylphenidate
suggests that this may be a useful approach [16]. The second issue is whether
the incident pain (pain on movement or on coughing in the postoperative
context) is simply the same pain at higher intensity or is something different;
is it a quantitative or a qualitative difference?
Quantitative increase in pain on movement - evidence from postoperative
pain
The evidence from postoperative pain suggests a quantitative difference
between pain at rest and pain on movement; more analgesia is required to
control the pain on movement, with the increased risk of adverse effects, but
the pain can be controlled, at least by regimes which incorporate all three WHO
ladder rungs, and particularly with the addition of local anaesthetic and
steroids [17]. The studies from this Copenhagen group have shown that none of
the individual rungs of the WHO ladder can achieve this degree of incident pain
control on their own. It is only by using combinations that they have made
progress. The ability to maintain pain intensity scores of zero on coughing for
eight days after colonic resection is remarkable [17]. The regime used was
methylprednisolone 30 mg/kg iv before surgery, thoracic epidural infusion of a
combination of local anaesthetic and morphine, intrathecal (intraoperative)
local anaesthetic and indomethacin 100 mg iv 8 hrly with oral indomethacin as
required.
For incident pain in cancer the message must be that if the incident pain is a
predictable quantitative increase of the pain at rest, then we should think of
combinations of the rungs of the ladder, and of the use of local anaesthetic
and steroids when that fails, by oral, subcutaneous [18] or epidural [19]
routes. At first sight the Copenhagen regime appears to be way beyond what
might be practical for cancer pain patients. The reality, however, is that
epidural infusion of combinations of local anaesthetic and opioid is already
being used outside hospital, and ten years ago few would have thought that
practical. RCTs of combinations are notoriously difficult to conduct in any
patient group, let alone epidural combinations in cancer pain patients, but
they are badly needed. With an epidural catheter in place it is easy for
booster doses to be given in response to episodic increase in pain intensity.
The issue is not the technical feasibility, but whether it is an effective
method and more appropriate than the alternatives.
Incident pain: qualitative increase in pain
The classes of drugs used commonly are antidepressants, anticonvulsants
and steroids. The RCTs of these 3 classes have been in chronic non-cancer pain,
and have focused on neuropathic pain syndromes, not on incident pain. The
importance of these drugs in that incident pain which is qualitatively
different from pain at rest is often of the neuropathic type. It is important
then to know whether or not the pain responds to these classes of drugs.
The recent randomised controlled studies of antidepressants have reinforced the
messages that first generation tricyclic antidepressants are more effective as
analgesics in this context than the more selective antidepressants, that the
relief and its duration do not depend on the quality of the pain (burning,
shooting etc.), and that the relief can be obtained well within the first week
[20, 21, 22, 23]. It is possible therefore to increase dose quickly to achieve
an adequate trial of antidepressants without wasting time which is precious to
the patient. Systematic review of anticonvulsants [24] showed similar benefit
and risk levels to antidepressants [25]. There is little evidence on which to
recommend one anticonvulsant rather than another. The oral (and intravenous)
use of the local anaesthetics is based on good animal studies, but again we do
not have adequate RCT evidence to recommend their use in man [26]. The use of
steroids remains an enigma. In postoperative pain the analgesic effect of
steroids has been shown. Widespread use of steroids in palliative care to
control pains resistant to other therapy has not been subjected to the
customary trials in non-cancer pain, because of concern about adverse effects.
The use of steroids is therefore based on experience. The most recent
development is the idea that ketamine, as a clinically available antagonist at
the N-methyl-D-aspartate receptor complex, may be useful in this type of pain.
Three recent studies support this idea, and we hope that full-scale RCTs will
show whether or not this is a useful ploy [27, 28, 29].
The place of opioids in the treatment of this qualitatively different pain
remains problematic. Doses of oral opioid adequate to control the incident pain
may be excessive when the incident pain stops. This begs the question as to
whether opioids can control this type of pain. The evidence suggests that, for
some patients at least, they can [13], and that all patients should have an
adequate trial of opioids. The problem remains that adverse effects may be
overwhelming in the absence of the incident pain. If opioids could be given
rapidly in response to the presence of the incident pain, were effective, and
did not remain active when the incident pain had gone, then they would be
appropriate.
Giving opioids as rapid 'boosters' in this context requires the use of routes
of administration other than oral, because speed of onset of effect is too slow
orally. Subcutaneous, intramuscular and intravenous routes can all provide
faster speed of onset of effect than oral opioids, but with an inevitable
increase in the logistic complexity. Bolus, infusion or patient-controlled
analgesia (PCA) techniques are all possible [30], but being possible as
alternatives to the oral route for cancer pain management in general does not
necessarily mean more effective or more appropriate for incident pain. With new
opioids such as remifentanil [31] which have a faster offset of effect than
those available currently, it should be possible to test whether or not fast
onset/fast offset opioids by injectable routes can improve incident pain
management.
The Future
It is striking that there is so little data from controlled trials to guide our
practice when the two incidence studies emphasise the importance of incident
pain. Some thoughts for these studies are that the pain must be characterised
well. Pain assessments need to be made at maximal pain, if predictable, and /
or with the movement(s) which provoke maximal pain. We would suggest that the
number of painful episodes should also be an outcome measure, with an
indication of the severity of the episodes. A measure of typical pain intensity
and typical pain relief [32] may be particularly valuable if the pain is
unpredictable. Just as important are the design requirements of the studies
[33]. Given that it is very difficult for any of us to do studies on
substantial numbers of patients in this group, it surely is time that we
combined forces to produce valid answers to worthwhile questions.
Red Herrings
Tolerance
Clinicians argue that tolerance to opioids, if it occurs, is driven by disease
rather than by pharmacological tolerance. The first problem is that tolerance
is used by some to mean any increase in dose, whereas others use it in the more
technical sense of an increased dose required to produce the same effect.
It is ingenuous to argue that opioid tolerance does not occur in man - fleeting
glimpses have been seen which echo the solid findings of both acute and chronic
opioid tolerance in animal models [34]. The classic Houde experiments showed
chronic tolerance when patients' analgesic response to a test dose was measured
before and after chronic dosing [35, 36]. The pragmatic issues are whether the
dose escalation required by some patients and producing difficult adverse
effects could be avoided (safely) by blocking a tolerance-induced need for dose
escalation, or (more simply) by changing opioid or indeed route of
administration.
Addiction
Clinical pain management has emphasised a difference between the clinical and
the laboratory pharmacology of opiates. It is as though there is one opiate
pharmacology when the opiate is used to counteract pain, and another when it is
not.
The respiratory depression which haunts prescribers in acute pain management is
seen readily in studies of volunteers who are not in pain. For patients with
opiate-sensitive pain, given appropriate doses of opiate, respiratory
depression is minimal [37, 38]. The balance between pain and opiate respiratory
effects is seen clearly in chronic pain. Patients maintained on oral morphine,
with no clinical respiratory depression, and who then receive successful nerve
blocks, must have their morphine dose reduced. Failure to reduce the dose will
result in respiratory depression [39, 40]. One explanation is that the
respiratory centre receives nociceptive input [41]. Presence of this input
counterbalances any respiratory depressant effect of the opiate. Absence of
this input, because of the successful nerve block, leaves the respiratory
depressant effect of the opiate unopposed.
The clinical message is that opiates need to be titrated against pain. Doses
higher than necessary for the relief of pain run the risk of respiratory
depression. Prophylactic use of opiates, infusion without regard to pain
experienced, doses greater than those required for analgesia (as in deliberate
ITU use to facilitate ventilation of a patient), use for purposes other than
analgesia (e.g. sedation), or use in non-nociceptive pain, thus all carry
potential risk. Concern about respiratory depression should not inhibit the
appropriate use of opioids, and that is to provide analgesia when the pain may
reasonably be thought to be opiate sensitive. A postoperative patient still
complaining of pain when the previous dose can be assumed to have been absorbed
needs more drug.
Similarly the drug-seeking behaviour synonymous with street addiction is not
found in patients after pain relief with opiates, either in childbirth, or
after operations or after myocardial infarction [42]. Street addicts are not in
pain. The political message is that medical use of opiates does not create
street addicts. Medical use may indirectly increase availability to those who
are already addicts, but restricting medical use hurts patients.
Adverse Effects
Specific adverse effects & metabolism
If an opiate has no specific advantage over morphine and has a specific
disadvantage, such as a troublesome adverse effect not found with morphine,
then there is little logic in choosing that drug in preference to morphine. Any
drug which produced fewer side-effects than morphine, at a dose which provided
the same degree of analgesia, would be an improvement. High dose fentanyl in
cardiac anaesthesia causes less haemodynamic disruption than high dose
morphine. For most clinically important side-effects there is no comparative
evidence at equivalent analgesic doses to recommend any of the alternatives.
Single-dose postoperative studies showed a higher incidence of nausea and
vomiting with pethidine [43], and dysphoria (see below) with those mixed
agonist-antagonists which have a relatively high affinity for kappa and sigma
receptors [44]. As with non-steroidal anti-inflammatory drugs, the risk:benefit
ratio may be different at equianalgesic dosing within the same patient [45],
but we cannot predict these individual responses.
DYSPHORIA
Dysphoria occurs with all opiates, but the incidence varies widely between
drugs. Pentazocine, butorphanol and nalbuphine have this potential [46]. The
greater than 20% incidence with pentazocine and butorphanol contrasts sharply
with the 3% incidence seen with other opiates. There is little sense in using
an opiate which produces a higher incidence of dysphoria than morphine without
any compensating advantage.
TOXIC METABOLITES
Pethidine is metabolised to norpethidine which is toxic [47]. It causes tremor,
twitching, agitation and convulsions, and the incidence of these problems
increases with multiple dosing and in the presence of impaired renal function
[47, 48]. Morphine-3-glucuronide (M3G) may be a toxic metabolite. When this
becomes problematic is still unclear.
ACTIVE METABOLITE
Whereas diamorphine [49] and M3G [50] do not bind to opiate receptors,
6-monoacetylmorphine, morphine, morphine-6-glucuronide (M6G) and normorphine
do. Recent work on the metabolites of morphine has important clinical
implications [51, 52]. Quantitatively the most important active metabolite is
M6G, because M3G and M6G are the major metabolites of morphine in man [53, 54]
and because of the greater potency of M6G compared with morphine. In rats, M6G
is 45 times more potent than morphine intracerebrally and nearly 4 times more
potent subcutaneously [55]; intrathecal injection gave potency ratios been 10
and 20 [56]. M6G may contribute substantially to the analgesic effect of
morphine, in both single and repeated doses [57, 58, 59].
Diamorphine is a classical pro-drug. Without analgesic activity itself, it
initiates the "cascade" into the active 6-monoacetylmorphine, morphine and M6G.
Because of the speed of these reactions, there is no clinical advantage over
morphine by oral or intramuscular routes, either in terms of greater analgesic
efficacy or of improved mood [60, 49]. This does not exclude advantage from
intravenous, spinal or other routes.
PATHOPHYSIOLOGY
Unexpected degree and duration of effect can be obtained with morphine, codeine
and dihydrocodeine when they are used in patients with severely impaired renal
function [61]. Cumulation of M6G is the probable explanation of this
phenomenon. Prolonged respiratory depression has been reported in man in
association with negligible plasma concentrations of morphine but with very
high concentrations of M3G and M6G [62]. Glucuronidation of morphine is altered
little in hepatic failure [63], but in pre-coma kinetics [64] and dynamics
[65] are altered.
Problems should arise only if a fixed-dose schedule is used without taking
account of renal function, or without adequate titration against pain
intensity. Drug doses should be decreased markedly if creatinine clearance is
less than 30 ml min-1. With less severe renal dysfunction the potential problem
emphasises the need for careful titration, remembering that renal function
deteriorates with advancing age.
NAUSEA AND VOMITING AND CONSTIPATION
It has been very difficult to obtain data on adverse effect incidence from
randomised studies. The recent paper by Moulin et al [66] showed that a third
of the forty patients experienced adverse effects under this heading.
Prescribing Opioids in Chronic Non-cancer Pain
The argument that patients in pain who do not have cancer should not be given
opioids is of great concern. If opioids provide pain relief for a patient, and
there is no other effective remedy, who is to decide that the patient should be
denied pain relief and for what reason? Obviously not all patients with
non-cancer pain should be treated with opioids. There are, however, of a small
number of patients in whom opioids are the only effective remedy. It is
the right of these patients to obtain effective relief, and of their doctors to
prescribe such relief for them, which should be upheld.
The reasons given commonly for withholding opioids in non-cancer pain include
concern for the patient and concern for the community. The concern for the
patient is that opioids may make the patient a drug addict and that opioids may
have other adverse effects on the patient's health. The concern for the
community is that opioid prescription to this patient group will perforce lead
to an increase in street addiction. There is no evidence that patients with
chronic non-cancer pain treated with opioids become addicts or that their
health is impaired. There is no evidence that such medical availability has any
impact on street addiction. Indeed there is evidence to the contrary. When oral
opioids began to be used in Sweden to control cancer pain, this increasing
medical availability had no impact at all on street addiction [67].
A recent review of the evidence for the treatment of cancer pain [68] is
equally valid for opioid prescribing in other pain contexts. The 45 authors, 12
pharmacologists, 10 physicians, 8 pain clinic doctors, 7 psychologists, 3
lawyers, 3 experts from drug addiction and 2 experts in biomedical ethics,
could find no medical, psychological, legal or ethical reason to withhold
opioids in cancer pain. What then is the difference in pain not due to cancer?
The obvious difference is in potential duration of treatment. The longitudinal
surveys published to date suggest no difference between the two populations of
patients.
The proportion of patients with chronic non-cancer pain for whom opioids might
be considered is small, because not all of these patients obtain relief from
opioids, some patients are psychologically unsuitable, some patients do not
want to take opioids and other doctors involved in the patients' care may
oppose opioid use. For those in whom we are considering opioid treatment we use
the following guide-lines:
-
All other relevant treatments have failed
-
The pain is shown to be relieved by opioids
-
The patient, after clear explanation and discussion with family and family
practitioner, is willing to take opioids
-
Other doctors involved in the patient's care agree with opioid
prescription
-
Appropriate follow-up
Those who oppose opioid prescription should make their motives clear. There is
no evidence to support the reasons which they advance. In the absence of such
evidence we hope that the rights of that small number of patients for whom
opioids are the only effective remedy to obtain relief, and the right of the
doctor to prescribe, will be protected.
Clearly it is neither necessary nor desirable to subject patients with terminal
disease who are obtaining effective relief from opioids to such a battery of
tests. Problems do arise, however, in which this approach may help. The first
is the general case when relief is ineffective and it is unclear to the
clinician whether or not better relief can be achieved by a higher dose without
intolerable or unmanageable adverse effects. This is equivalent to the question
'Can a poor or moderate response be converted to a good response by using a
higher concentration?' The second is specific to the vexed question of opioid
prescription in chronic non-cancer pain. Such prescribing is viewed as
potentially dangerous for both the patient and society [69, 70], although
published evidence suggests that neither belief is valid [68]. Opioids should
be considered for patients for whom there is no other effective remedy, in whom
opioids are effective, and given that both the patient and the patient's
doctors agree [71].
FOR MORE INFORMATION:
Visit THE OXFORD PAIN INTERNET SITE







